SunGreenH2’s nano-scale engineering could double green hydrogen production – TechCrunch

If there is ever going to be a national hydrogen economy, we’re going to need to change a few things. One of them is how we produce hydrogen itself, the vast majority of which comes from fossil fuels. SunGreenH2 brings a nanotech-level advance to green hydrogen production, supercharging electrolysis so orders of magnitude more hydrogen can be cleanly made directly from water.
Hydrogen is in use all over the place, but the lack of a scalable, green option for producing it has slowed its adoption. What’s the point of having a hydrogen battery system for renewables if you have to source that hydrogen from natural gas, oil and coal?
The answer is the process of electrolysis, which separates water molecules into their constituent atoms, producing hydrogen and oxygen. Sounds great, but it takes a lot of energy and expensive elements like platinum to produce the catalysts and membranes that make up the tech sandwich of any modern electrolyzer cell. So green hydrogen is several times more expensive than dirty hydrogen, and harder to make to boot.
In comes SunGreenH2, which has made a major improvement to a crucial part of those cells: the electrode. The company claims that it can double hydrogen production, lower the cost, and reduce reliance on platinum and other rare elements all in one swoop.
“We are nanostructuring a proprietary alloy-based cathode that doubles the surface area available for the reaction,” said CEO and co-founder Tulika Raj. “That doubles the amount of hydrogen produced right there. But that’s not enough, you need to to be scalable and manufacturable. So it doesn’t use precious metals — in this case we are reducing the amount needed by 30x.”
She said that practically all modern electrolyzers can use this improved electrode. “The idea is to left the entire industry,” she said.
The nanostructure surface is the primary advance, and you can see a scanning electron microscope image of it here:
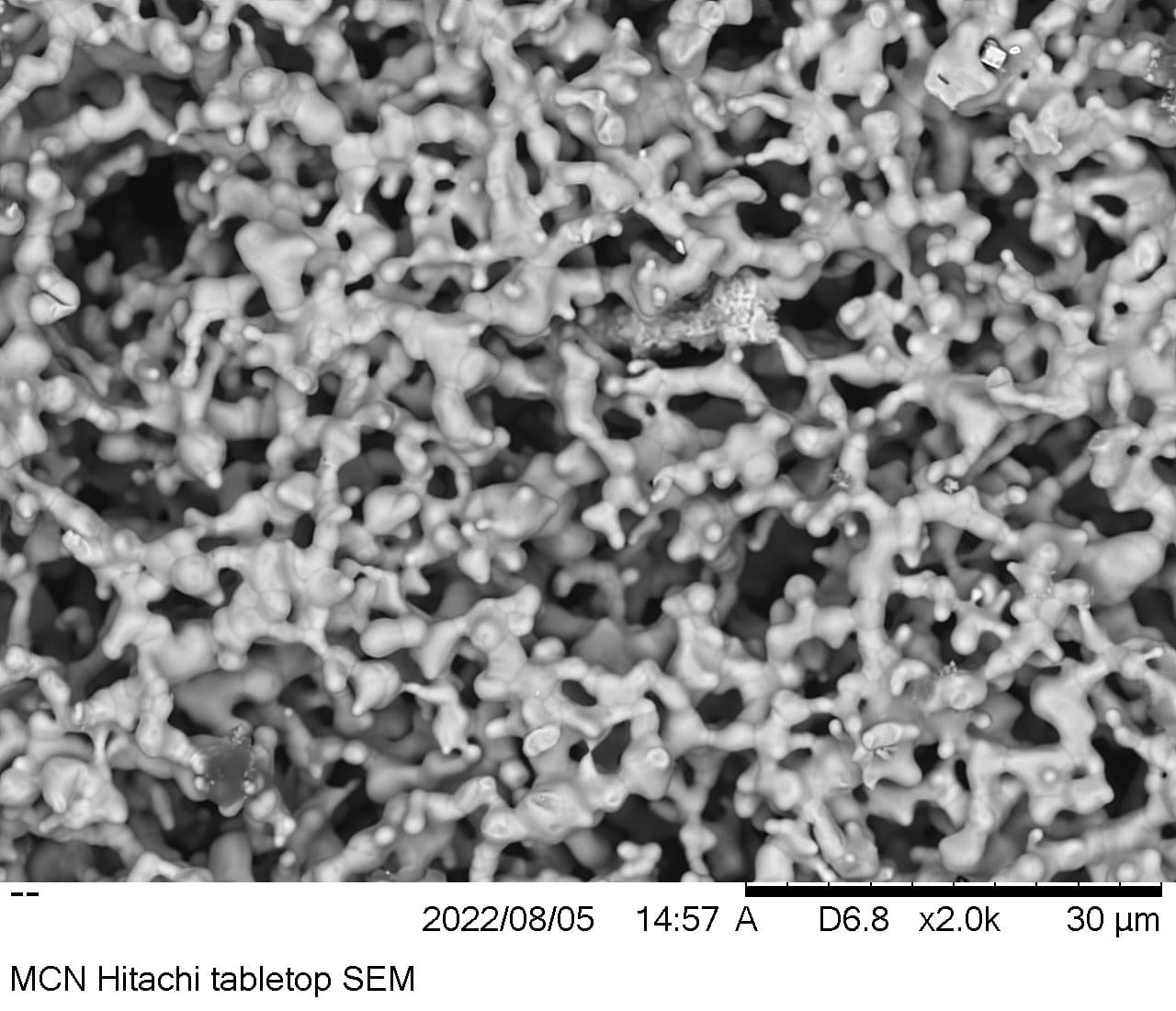
Image Credits: SunGreenH2
It looks a bit chaotic, but the material is carefully engineered to interact with the other chemical components of the electrolysis process (mainly water and a catalyst), and by forming a sort of 3D sponge structure rather than a flat or rough one, it doubles the surface area that reaction can happen on.
One weakness of nanostructures is that they’re very fragile and prone to degradation, reducing their effectiveness (however miraculous) over time. SunGreenH2 has gotten out ahead of this flaw with “the novel concept of sacrificing catalyst.”
“Increasing the surface area and tuning the crystallinity of the deposited materials results in improvement of the performance and stability, respectively, while using the sacrificing catalyst results in significant enhancement of the lifespan of the main catalyst,” Raj explained. “In current/existing technology, due to corrosion of the catalyst, the performance of the electrode reduces. In the technology developed by SunGreenH2, corrosion of the sacrificing catalyst results in enhancement of the surface area, which compensates for the impact of corrosion.”
SunGreenH2 cofounders Tulika Raj (left) and Saeid Masudy Panah. At right, a prototype electrolysis stack with the company’s tech in the middle somewhere.
In other words, this second material pervades the structure, and its corrosion process (by some means beyond my understanding) keeps the main catalytic surface fresh.
The company has raised a $2 million seed round led by SGInnovate, with participation from Vinci BV, Cap Vista, Entrepreneur First, SOSV’s HAX, she1K, and Apsara Investments. Raj said that this money will go to setting up their first manufacturing facilities in Melbourne, to meet the demands of their early partners, which are confidential but include major energy concerns in the E.U., U.S. Canada, Japan, and Singapore.
Though the intention at first is to sell the electrode component separately, next year it plans to partner with system integrators to produce whole electrolysis stacks, and eventually with larger companies to produce more end-to-end solutions. “We can also apply our platform technology to fabricate similar green transition materials, i.e. electrodes, PTLs and bipolar plates for fuel cells, electrodes for batteries and photoelectrodes for direct solar to hydrogen panels,” Raj said.
All that depends on their success in these early days, but if a green hydrogen revolution is going to happen, the industry will have to start embracing technologies like this one sooner rather than later.
Recent Posts
- Lindungi Kulit dengan Skincare yang Sesuai Kategori Usia
- Respons Gibran Usai Luhut Minta Prabowo Jangan Bawa Orang Toxic
- Comment: Now is the time to reflect the excitement felt by our customers
- Sejarah Pramuka Indonesia dan Tingkatannya
- Accor unveils its first Handwritten Collection address in the United States
Recent Comments